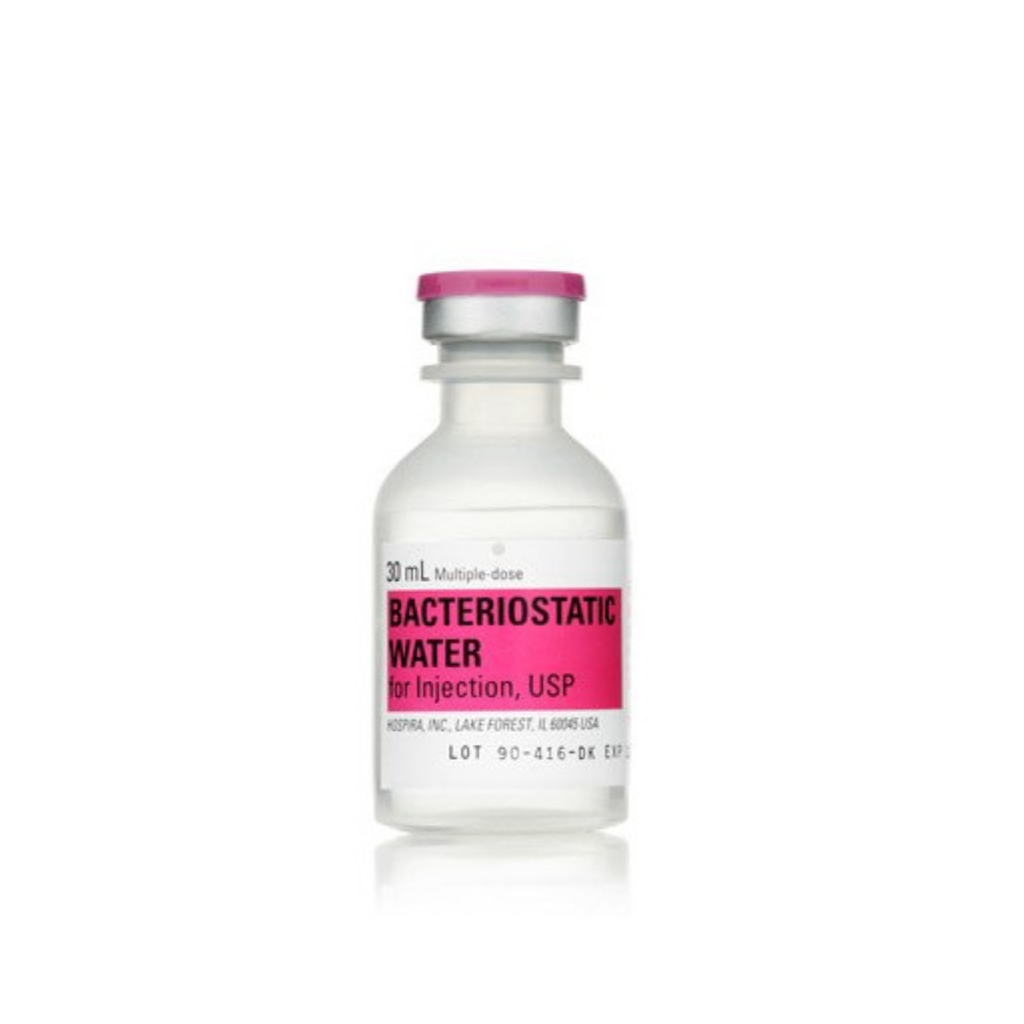
Bacteriostatic (BAC) Water
Limit Of 4 Per Order
The products available here are intended strictly for research and laboratory use only.
They are not approved for human or animal consumption.
These materials are not medicines or drugs and have not been evaluated or approved by the
U.S. Food and Drug Administration (FDA) for the diagnosis, treatment, cure, or prevention
of any disease or medical condition.
Any form of bodily administration or ingestion is strictly prohibited by law.
buy more, save more!
Price:
$19.00
Availability: In stock
Pfizer Hospira Bacteriostatic Water (30 mL)
Sterile multiple-dose diluent containing 0.9% benzyl alcohol. Supplied exclusively for laboratory research use.
Overview
Bacteriostatic Water for Injection is sterile water containing 0.9% benzyl alcohol to inhibit bacterial growth in a multi-use vial format.
Each lot is third-party tested to verify identity and purity. Product labeling may vary slightly when sourced from Canadian Pfizer Hospira supply lines during U.S. shortages; formulation and quality standards remain the same.
Key Features
- Third-party tested for mass and purity
- Sterile, multiple-dose plastic vial (30 mL)
- Preserved with 0.9% benzyl alcohol
- Lot numbers printed for traceability
- Protective packaging to help maintain vial integrity in transit
Compliance & Use
- Intended exclusively for laboratory research, assay development, and related investigative use
- No medical, diagnostic, or therapeutic applications are intended or implied
- Handle using aseptic laboratory technique per your facility SOPs
Specs
What’s Included
- Pfizer Hospira Bacteriostatic Water, 30 mL multiple-dose vial
- Lot and expiry printed on label
Trademarks are the property of their respective owners. No clinical claims are made or implied.